Mission
MDMI is focused on making the medicines development process more efficient and less resource intensive by achieving the full potential of medicine development tools (MDTs). The long-term goal of the Initiative is to create a modern approach that brings all stakeholders and the technological and scientific advances of recent decades fully to bear.
Much like the technology industry’s transition to agile, user-facing software development, this modern medicines development ecosystem will include a transparent process to collect comprehensive, detailed needs and expectations from all stakeholders up front, routine interim opportunities to gather stakeholder feedback as the process progresses, and the ability to transparently shift direction as interim feedback is collected – all with the goal of more rapidly and efficiently delivering medicines that are more valuable to more stakeholders.


What are Medicines Development Tools?
The Initiative is intended to capture and consider medicines development tools as broadly as possible, including both actual measurable markers and endpoints as well as strategic/tactical approaches such as continuous development. Other medicines development tools include model-informed drug development, biomarkers, and patient-focused drug development.
In the U.S. regulatory environment, these are commonly referred to as “Drug Development Tools.” We have adopted the more global phrase “medicine development tools.”
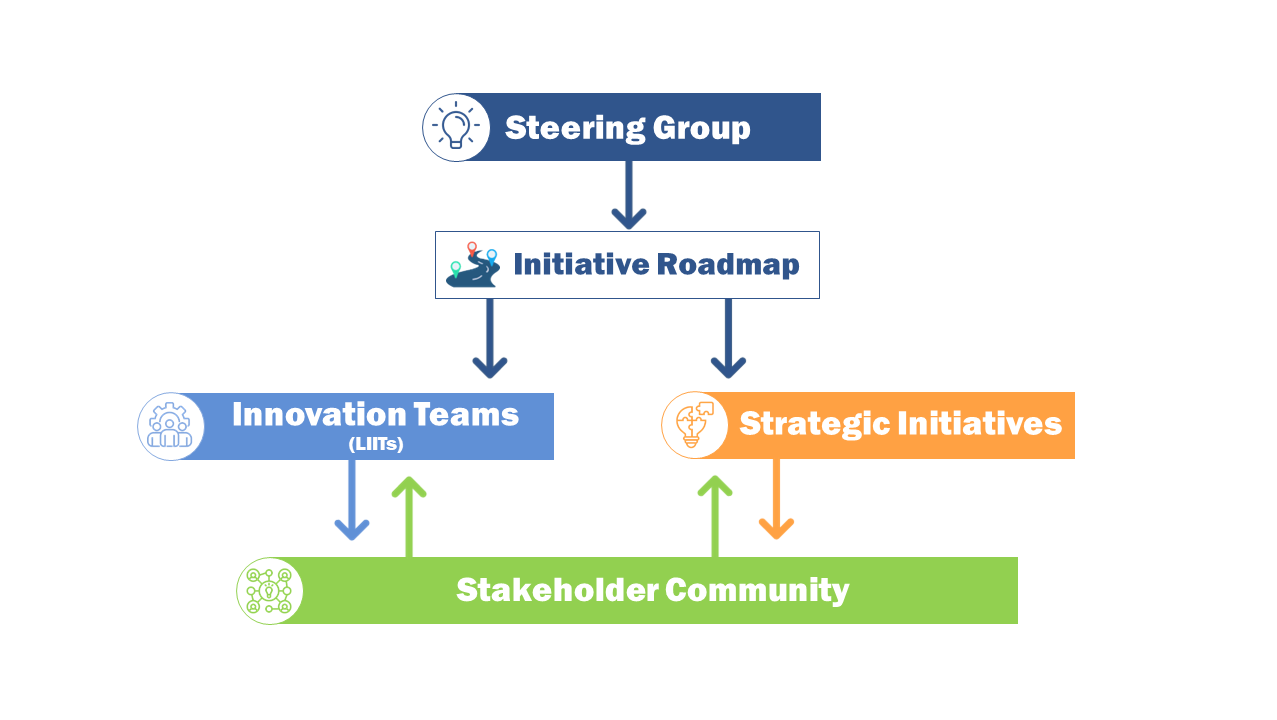
Steering Group
A Steering Group representing a diversity of experiences and perspectives provides strategic direction and leadership to MDMI in furtherance of its mission.
The Steering Group is comprised of the following individuals: